Brosmed receives CE mark approval for POT™ PTCA balloon catheter
Sittard, Netherlands [May 24, 2021] –Brosmed, a medical device company developing and commercializing innovative interventional treatment systems for patients with coronary and peripheral disease today announced she received CE Mark approval in the European Union for its POT™ PTCA balloon catheter, a dedicated balloon for the proximal and distal optimization technique(PDOT). This is the first-ever balloon product applied to POT and DOT technology .
The proximal optimization technique (POT) has been proposed as a strategy to improve the results of stent scaffolding at the lesions. It is a straightforward technique whereby a short, appropriately-sized balloon is inflated in the main vessel just proximal to the carina. The technique has several advantages: it reduces the risk of side branch compromise related to shifting of the carina, it improves stent apposition in the proximal main vessel, and it facilitates side branch access after main vessel stent implantation.

The POT™ PTCA catheter is a non-compliant balloon designed with extra short balloon shoulders in order to reduce longitudinal balloon growth, minimizing the potential for vessel trauma outside the treatment area.
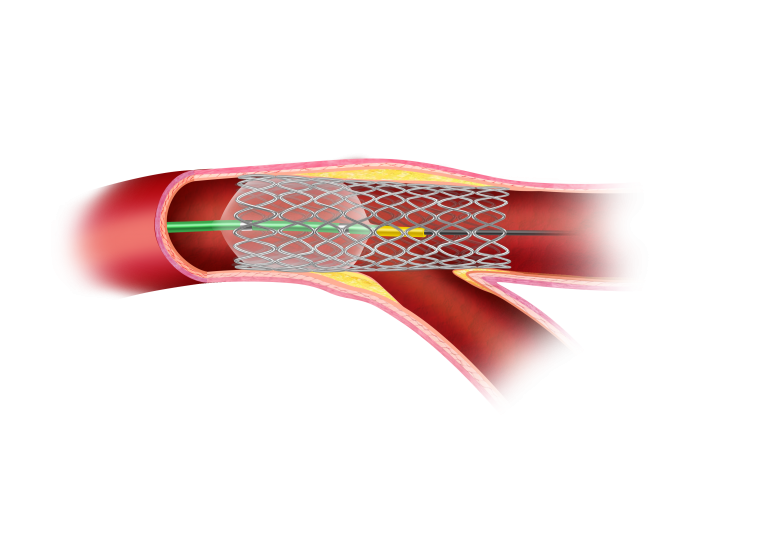
Stent under expansion may have immediate implications during bifurcation PCI. With POT™, workhorse stents can be evenly expanded to improve stent apposition.
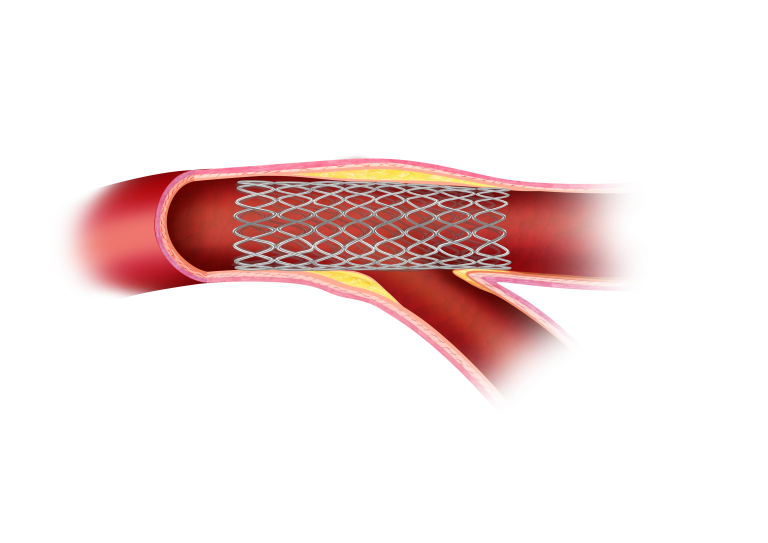
Pre-POT
After-POT
Prof. Shaoliang Chen, the inventor of POT™ PTCA balloon catheter said “Percutaneous coronary intervention using drug-eluting stent is a mature treatment for patients with obstructive coronary artery disease. However, the metal stents could not be sufficiently expanded for most cases. This requires the performance of post-dilation in order to achieve proximal , in-stent segment, and distal optimization. Proximal optimization technique (POT) and distal optimization technique (DOT) are commonly associated with complications, such as dissection, hemetoma, or vascular rupture, mainly because of longer shoulder of a non-compliant balloon which is out of stent edge. A real POT balloon innovated by Brosmed has provided the final solution for either POT or DOT. POT PTCA balloon catheter offers tremendous promise and adds a state-of-the-art coronary balloon to Brosmed leading products portfolio.”
“ We are pleased to expand our coronary interventions portfolio to include specialty catheters for complex coronary interventions.” said David Camp Business development VP of Brosmed . “Brosmed remains committed to providing innovative devices to our physician and their patients and we will strive to continue to develop and introduce devices that address common clinical challenges with existing products.”
About BrosMed Medical
BrosMed Medical develops, manufactures and markets medical devices primarily used by interventional cardiologists, radiologists and vascular surgeons in angioplasty procedures and other minimal invasive devices.
We provide solutions to our customers through innovative design, effective supply chain management, world-class manufacturing, and flexible channels of distribution and through the development or acquisition of innovative technologies.
Learn more about Brosmed at http://www.brosmed.com

